Clinical Study – IMM-101 Phase II data
IMM-101 is a broad spectrum immunomodulator
IMM-101’s diverse immune related mode of action and the potential safe promotion of a broad systemic innate and adaptive immune response provides a strong rationale for targeting a range of cancers. Immodulon is initially focusing on the high unmet needs of pancreatic cancer patients with further opportunities in other cancers including melanoma and colorectal cancer.
“IMM-101 uniquely boosts the innate immune response, enhances T-cell mediated immunity and suppresses the chronic inflammatory response. It’s the only agent that can do this”
IMM-101 compelling phase II pancreatic cancer data
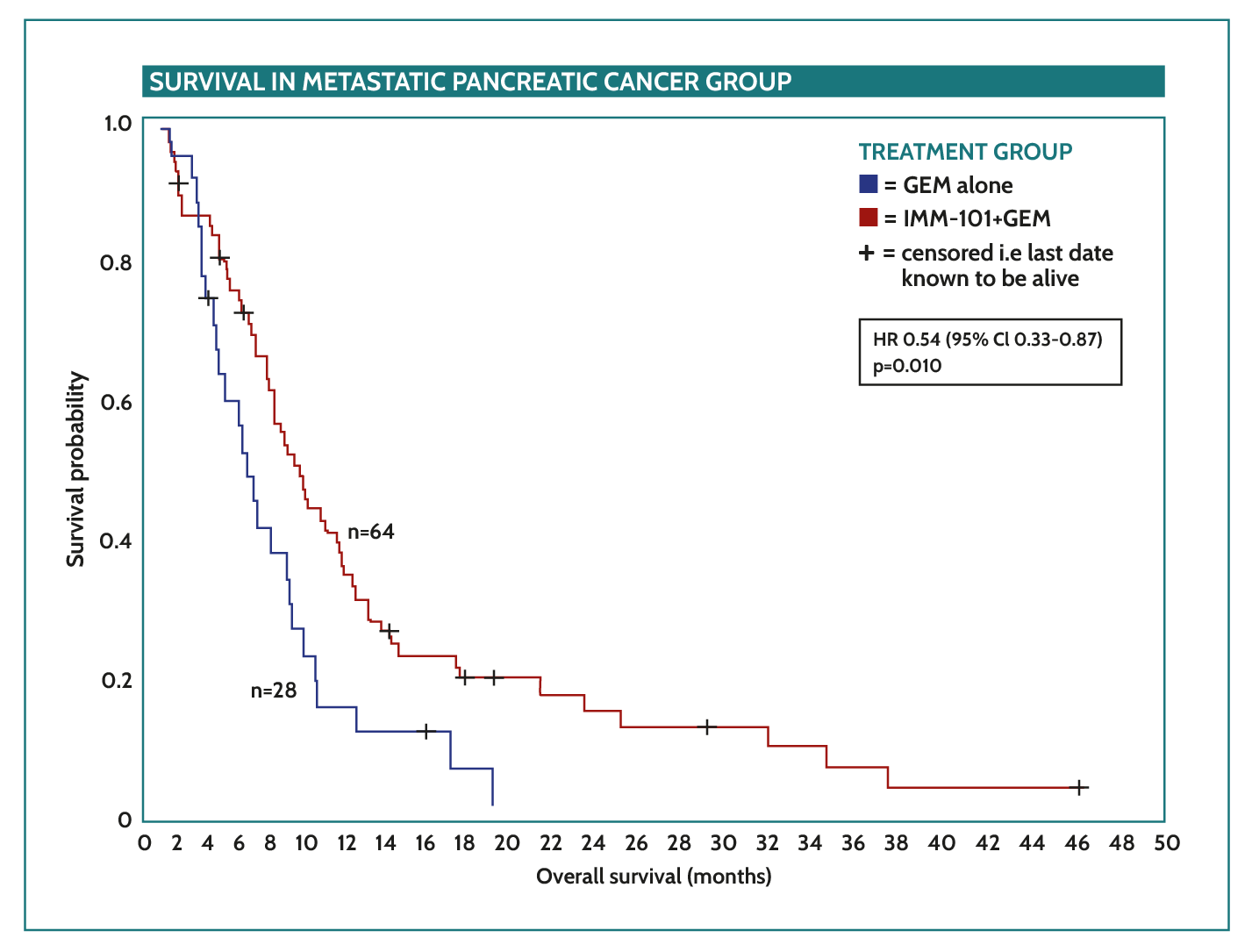
- Median overall survival of metastatic pancreatic cancer patients receiving gemcitabine + IMM-101 improved from 4.4 months to 7 months (p<0.01)
- Performance Status 2 (PS2) patients performed equally as well as PS1 and PS0 patients
Safety
- IMM-101 was well tolerated and with no added toxicity
Registration Study
Immodulon is planning to advance IMM-101 in a pivotal study in metastatic pancreatic ductal adenocarcinoma (mPDAC) patients . The study will evaluate IMM-101 combined with the current standard of care, Gemcitabine/Nab-Paclitaxel (Gem/Nab-P), versus Gem/Nab-P alone. The study aims to demonstrate improved median OS in elderly patients with mPDAC and in younger patients for whom a FOLFIRINOX regimen is not suitable.
